
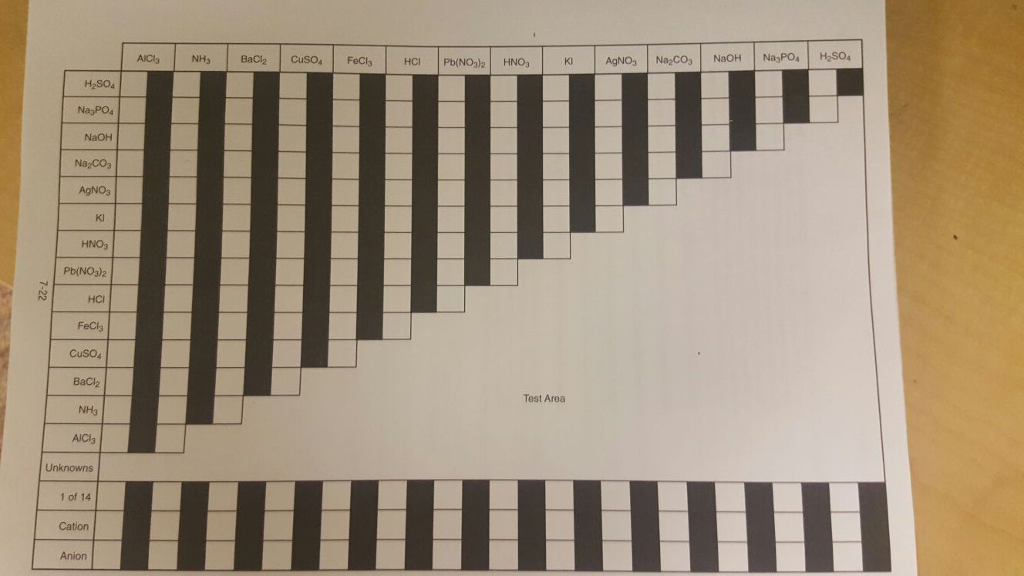
Set up a table similar to the one shown below.Yes, there will be the formation of precipitate in this reaction.Usually involves the mixing of two clear solutions to produce an insoluble When an aqueous solution containing Ag+ ions is added to an aqueous solution of sodium carbonate (Na2CO3), a white precipitate will form.

When silver nitrate reacts with sodium sulfate, then the products that are formed Does Ag+ and Na2CO3 form a precipitate? Yes, there will be the formation of precipitate in this reaction. What type of reaction is Na2CO3 AgNO3?ĭouble displacement reaction Does na2so4 and AgNO3 form a precipitate?
Here, barium chloride (BaCl2) is added to sodium carbonate (Na2CO3). When 0.25M solution of silver nitrate (AgNO3) is added to 1M solution of sodium hydroxide (NaOH), dark brown precipitate composed of various hydrated silver oxides (Ag2O xH2O) is produced What reacts with Na2CO3 to form a white precipitate? Does NaOH and AgNO3 form a precipitate?ĭescription: AgNO3 reacts with NaOH. What forms a precipitate with AgNO3?įor example, when an aqueous solution of silver nitrate (AgNO3) is added to the aqueous solution of sodium chloride (NaCl), a white precipitate of silver chloride (AgCl) is formed that is indicated by the following chemical reaction. What happens with Na2SO4 and AgNO3?Įxplanation: Silver nitrate, AgNO3, will react with sodium sulfate, Na2SO4, to produce silver sulfate, Ag2SO4, an ionic compound that is considered insoluble in aqueous solution, and aqueous sodium nitrate. Na2CO3 + 2 AgNO3 Ag2CO3 + 2 NaNO3 – Balanced equation | Chemical Equations online! What is the net ionic equation for AgNO3 K2SO4?Įxplanation: Silver nitrate, AgNO3, will react with sodium sulfate, Na2SO4, to produce silver sulfate, Ag2SO4, an ionic compound that is considered insoluble in aqueous solution, and aqueous sodium nitrate.

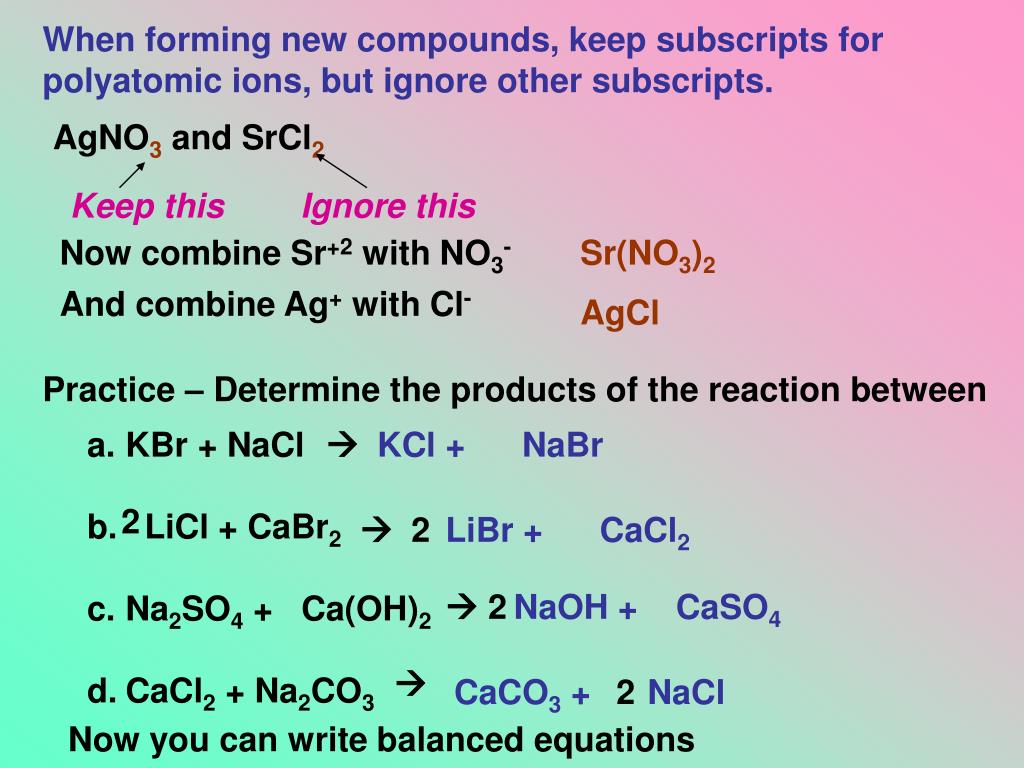
When silver nitrate reacts with sodium sulfate, then the products that are formed What happens when na2co3 reacts with AgNO3? Will Na2SO4 and AgNO3 form a precipitate? The Na in Na2CO3 and the Ag in AgNO3 switch places in the reaction. Na2CO3 + AgNO3 is a double displacement reaction. This means that we will split them apart in the net ionic equation. What happens when AgNO3 reacts with Na2SO4?Ģ AgNO3 + K2SO4 Ag2SO4 + 2 KNO3 – Balanced equation | Chemical Equations online! What is the net ionic equation for AgNO3 na2so4?īoth Na2CO3 and AgNO3 are considered strong electrolytes and will dissociate completely. What is the net ionic equation for AgNO3 and Na2CO3?Įxplanation: Silver nitrate, AgNO3, will react with sodium sulfate, Na2SO4, to produce silver sulfate, Ag2SO4, an ionic compound that is considered insoluble in aqueous solution, and aqueous sodium nitrate. What is the net ionic equation for AgNO3 Na2SO4?Įxplanation: Silver nitrate, AgNO3, will react with sodium sulfate, Na2SO4, to produce silver sulfate, Ag2SO4, an ionic compound that is considered insoluble in aqueous solution, and aqueous sodium nitrate. What is the ionic equation for AgNO3 Na2SO4?Įxplanation: Silver nitrate, AgNO3, will react with sodium sulfate, Na2SO4, to produce silver sulfate, Ag2SO4, an ionic compound that is considered insoluble in aqueous solution, and aqueous sodium nitrate. What happens when AgNO3 reacts with k2so4?.Does Ag+ and Na2CO3 form a precipitate?.Does na2so4 and AgNO3 form a precipitate?.What reacts with Na2CO3 to form a white precipitate?.Does NaOH and AgNO3 form a precipitate?.What is the net ionic equation for AgNO3 K2SO4?.What happens when na2co3 reacts with AgNO3?.Will Na2SO4 and AgNO3 form a precipitate?.What is the net ionic equation for AgNO3 na2so4?.What happens when AgNO3 reacts with Na2SO4?.What is the net ionic equation for AgNO3 and Na2CO3?.What is the net ionic equation for AgNO3 Na2SO4?.What is the ionic equation for AgNO3 Na2SO4?.


 0 kommentar(er)
0 kommentar(er)
